Introduction
Understand the potential for phototoxicity following topical exposure of your individual ingredients, chemicals or pharmaceutical products.
Phototoxicity assessment is in our portfolio of in vitro toxicology services for the cosmetics/personal care, chemical or pharmaceutical industry. Cyprotex delivers consistent, high quality screening data under non-GLP conditions.
Phototoxicity and its measurement in vitro:
- Phototoxicity is defined as a ‘toxic response from a substance applied to the body which is either elicited or increased (apparent at lower dose levels) after subsequent exposure to light, or that is induced by skin irradiation after systemic administration of a substance1.
- The assay is appropriate for pharmaceuticals, chemicals or cosmetics/personal care products which absorb in the wavelength range of 290-700nm, and are applied to, or can reach, the skin or eyes.
- Cyprotex’s phototoxicity service is a non-GLP screening assay performed using Balb/c 3T3 cells. The cells are incubated with increasing concentrations of the test article in the presence and absence of a non-toxic dose of UVA irradiation. Cytotoxicity is assessed via Neutral Red uptake.
Protocol
Phototoxicity Assay Protocol
Data
Data from Cyprotex's Phototoxicity Assay
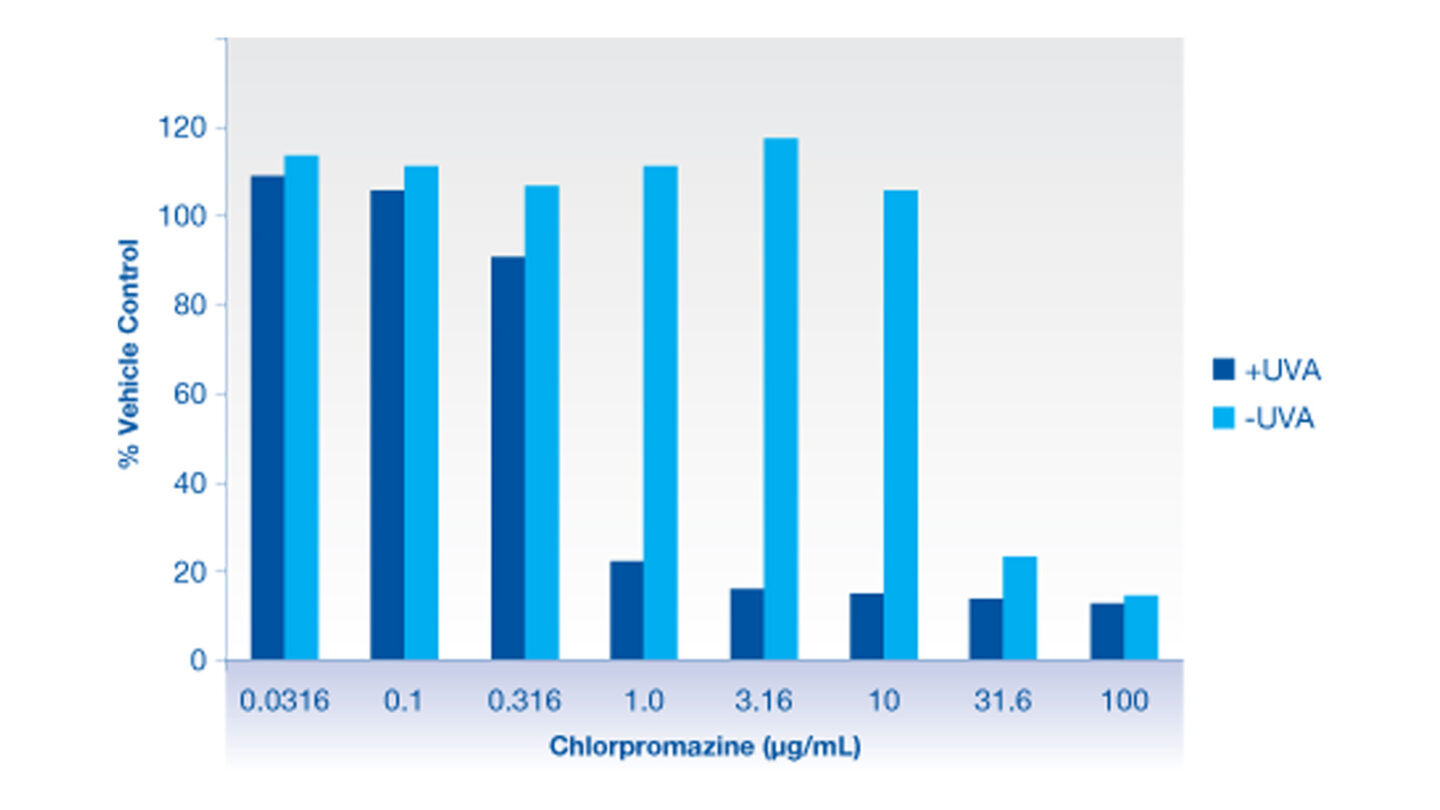
Figure 1
Graph illustrating neutral red uptake data for the positive control compound, chlorpromazine, in the presence and absence of UVA irradiation using 3T3 cells.
References
1) OECD Guideline for the Testing of Chemicals 432: In Vitro 3T3 NRU Phototoxicity Test; April 2004

