Introduction
Background information:
- Reactive metabolite formation is thought to be one of the primary causes of idiosyncratic adverse drug reactions, often associated with drug-induced skin, liver and hematopoietic toxicities.
- Reactive metabolites, formed via drug metabolism in the body, are electrophilic species which can bind covalently to macromolecules such as proteins and DNA, affecting their function and potentially leading to toxicity.
- Screening for reactive metabolite formation at an early stage in lead optimization is now common practice to minimize the risk of later stage failure, which is of considerable financial burden to the pharmaceutical industry1.
- Chemical trapping agents, such as reduced glutathione (GSH), potassium cyanide (KCN), and cysteine (Cys) can form stable adducts with many reactive species
- Reactive metabolites are typically electrophiles; soft electrophiles including quinoneimines, nitrenium ions, arene oxides, quinones, imine methides and Michael acceptors will conjugate with glutathione and cysteine, whereas hard electrophiles are generally charged species like alkyl carbocations and nitrenium ions and these can be trapped with potassium cyanide2.
- By using high resolution mass spectrometry, it improves detection of the conjugates and allows superior structural characterization. The process utilizes MSE data acquisition, mass detect filtering and post acquisition data mining.
- Cyprotex offers the reactive metabolite screen with the addition of stable labeled glutathione, potassium cyanide or cysteine in the presence and absence of nicotinamide adenine dinucleotide (NADPH). When incubated in a 1:1 ratio of unlabeled and labeled trapping agent an easily recognizable isotopic doublet allow for easy confirmation of reactive species. This additional diagnostic tool increases the robustness of identified conjugations.
Protocol
Reactive Metabolite Assessment Protocol
Data
Data from Reactive Metabolite Assessment
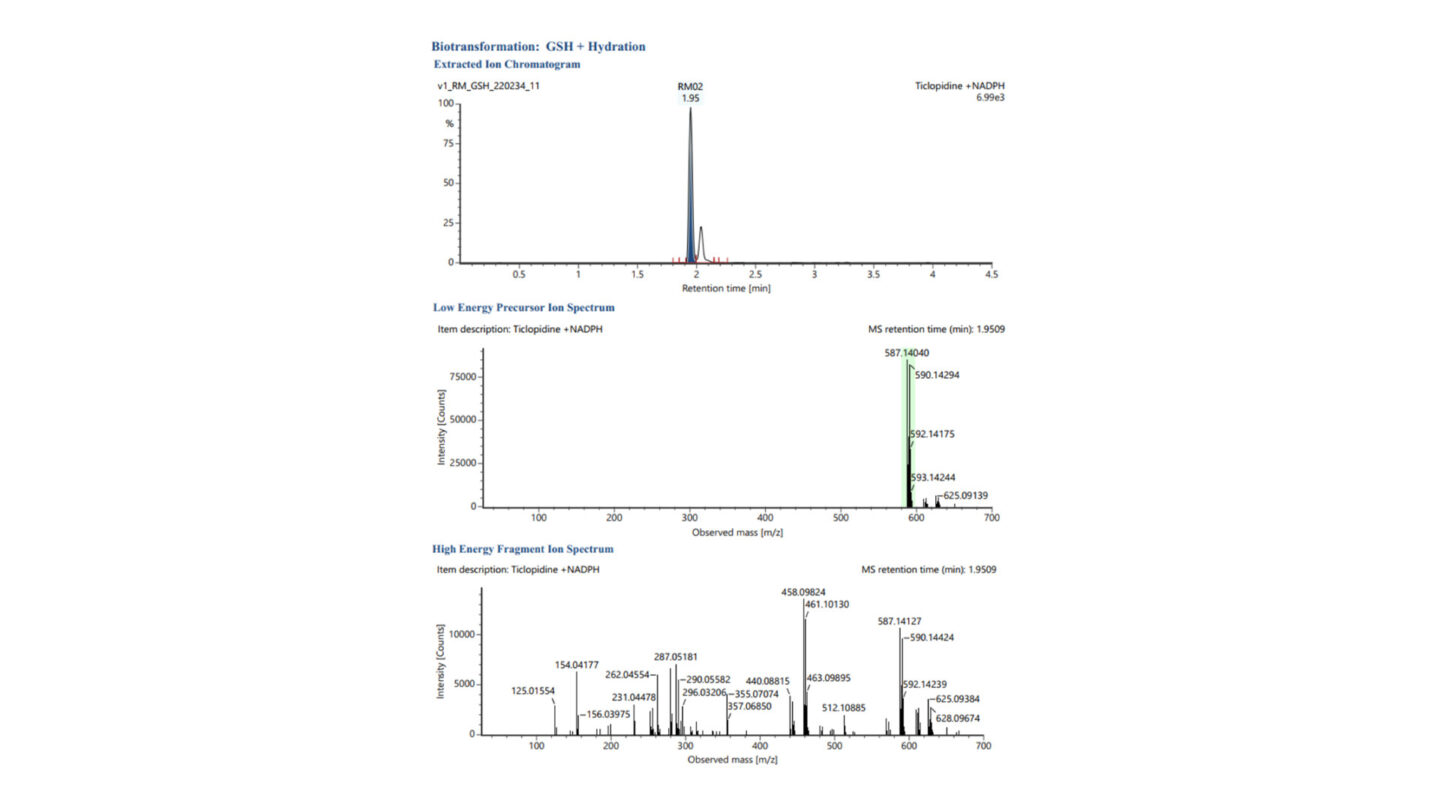
Figure 1
Representative extracted ion chromatogram (XIC) and low and high energy MSE spectra for GSH + Hydration (labelled RM02, m/z 587.1404 – highlighted green in low energy precursor ion spectrum) following incubation of ticlopidine with human liver microsomes, stable labelled and unlabelled glutathione, and NADPH.
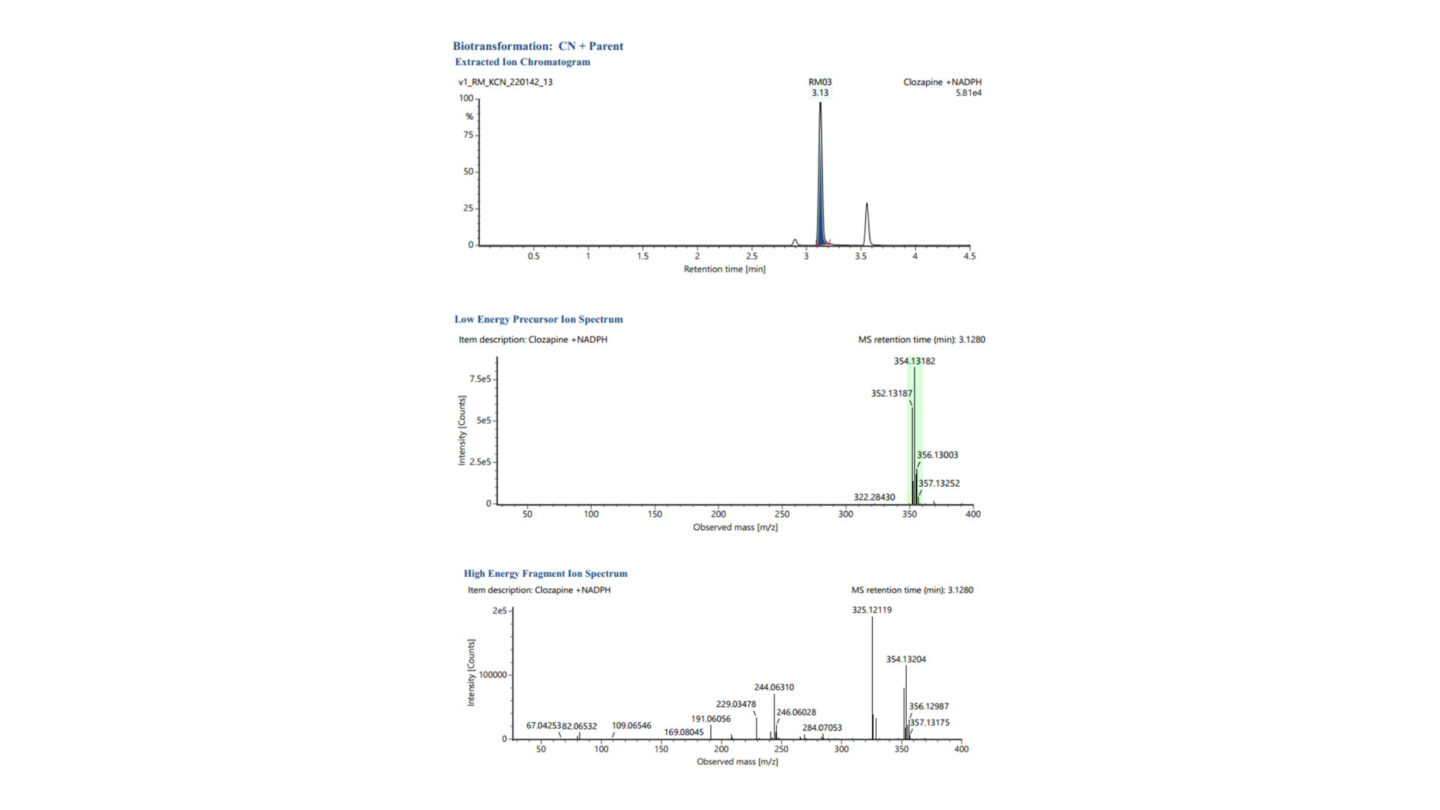
Figure 2
Representative XIC chromatogram and low and high energy MSE spectra for CN + Parent (labeled RM03, m/z 352.1314 – highlighted green in low energy precursor ion spectrum) following incubation of clozapine with human liver microsomes, stable label and unlabeled potassium cyanide, and NADPH.
References
1) Evans, DC. et al. Drug−protein adducts: An industry perspective on minimizing the potential for drug bioactivation in drug discovery and development. Chem. Res. Toxicol. 2004, 17(1), 3–16
2) Attia, SM. Deleterious effects of reactive metabolites. Oxid. Med. Cell. Longev. 2010, 3(4); 238-253
Downloads
- Cyprotex Mechanisms of Drug Induced Toxicity Guide >
- Cyprotex Reactive Metabolite Assessment Factsheet >

