The cell stress panel is a combination of endpoints that delivers a toxicity profile for novel therapeutics providing evidence of the toxicological mode of action. It consists of 36 biomarkers that represent cellular stress signalling pathways, organelle health and cellular cytotoxicity.
Cyprotex delivers consistent, high quality data with the flexibility to adapt protocols based on specific customer requirements.
Introduction
- Current in vitro preclinical strategies require ethical and regulatory consideration of animal use (3R's) and require human relevant data. This expedites new approach methodologies (NAMs) aimed at accurately predicting in vivo toxicities at therapeutically relevant in vivo concentrations1,2.
- Despite in vitro two-dimensional cellular assays lacking the complexity of the in vivo microenvironment, they provide a fast, economical readout for high throughput analysis3,4.
- 36 biomarkers are measured (figure 1) that represent 12 key cellular stress mechanisms including mitochondrial toxicity, gene regulation and inflammation. In addition, general cell health are monitored utilizing a variety of in vitro assays including the Seahorse platform, Promega kits and ELISAs, however, predominantly high content imaging (HCI) is used.
- It has been shown that the cellular stress panel can be used, together with other new approach methodologies, to identify chemical exposures that are protective of consumer health5.
Protocol
Cell Stress Panel Protocol
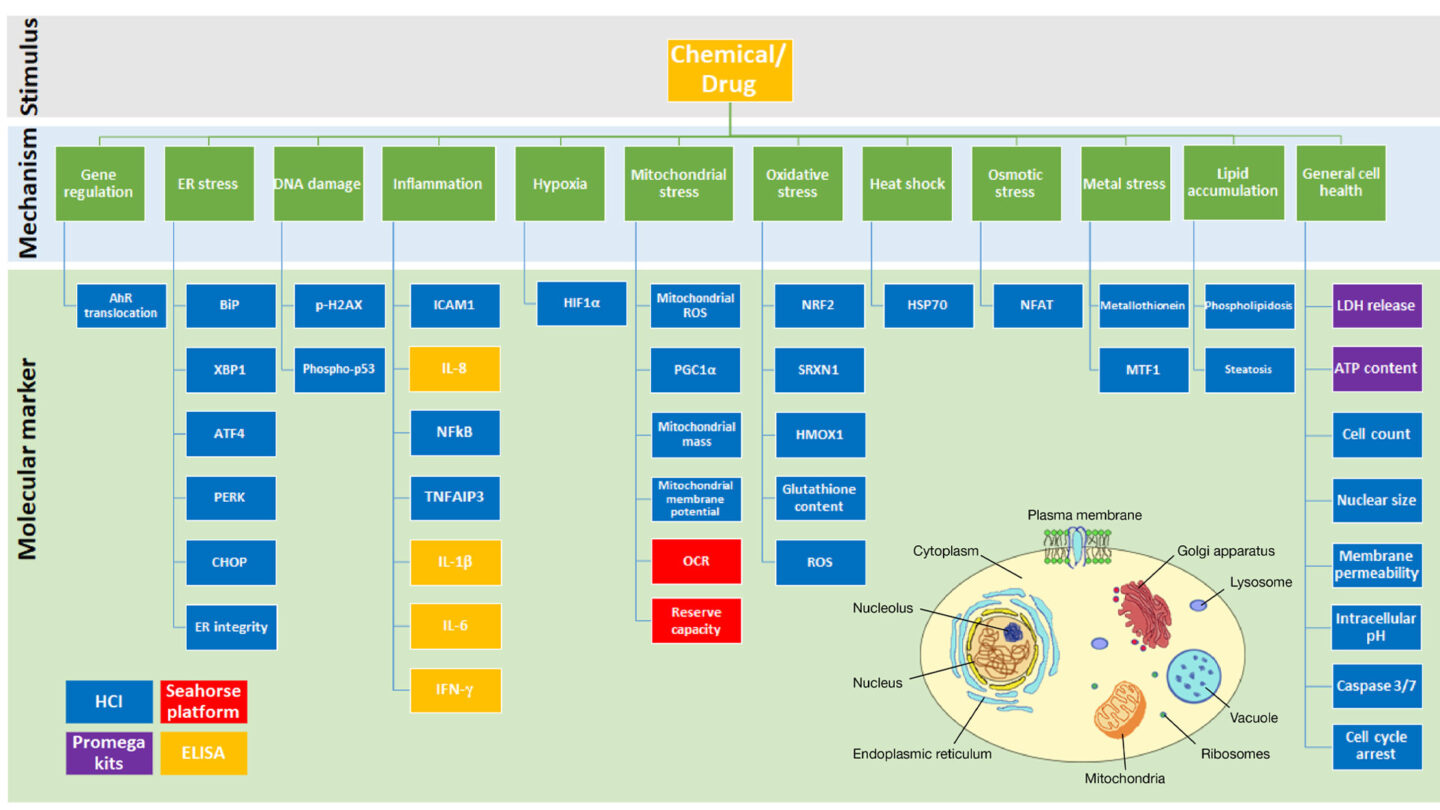
Figure 1
Cell stress panel overview summarizing the 12 cell stress mechanisms and their associated molecular markers detected within the cell stress panel assay.
Data
Data from Cyprotex's Cell Stress Panel
References
1) Dent M et al. (2018) Principles underpinning the use of new methodologies in the risk assessment of cosmetic ingredients. Comput Toxicol 7: 20-26
2) Middleton A et al (2017) Case studies in cellular stress: defining adversity/adaptation tipping points. Appl In Vitro Toxicol 3(2): 199-210
3) Campbell JL et al. (2012) Physiologically based pharmacokinetic/toxicokinetic modeling. Methods Mol Biol 929: 439-499
4) Moxon TE et al. (2020) Application of phisiologically based kinetic (PBK) modelling in the next generation risk assessment of dermally applied consumer products. Toxicol In Vitro 63: 104746
5) Hatherell S et al. (2020) Identifying and characterizing stress pathways of concern for customer safety in next generation risk assessment. Toxicol Sci 10.1093/toxsci/kfaa054

