Clinical Development to Commercial Manufacturing
Commercial Process Development (CPD) is a set of activities for optimizing bioprocesses following early-stage clinical development and in preparation for commercial manufacturing. CPD can be performed on either existing continuous or fed-batch antibody manufacturing processes. Existing fed-batch processes are converted to our integrated continuous manufacturing production line during CPD. This allows pharma and biopharma companies that have previously manufactured in fed-batch to launch their products with lower manufacturing costs, greater agility, and greater supply chain security to avoid drug shortages.
Watch Our Webinar On-Demand
A Roadmap to Commercial Launch with a Continuous Manufacturing Platform
Partners with projects that have previously utilized a fed-batch process typically request a feasibility study before transitioning to CPD. These studies assess the viability and practicality of implementing continuous production for a specific product.
Existing projects with continuous manufacturing processes proceed directly into CPD.
Commercial Process Development
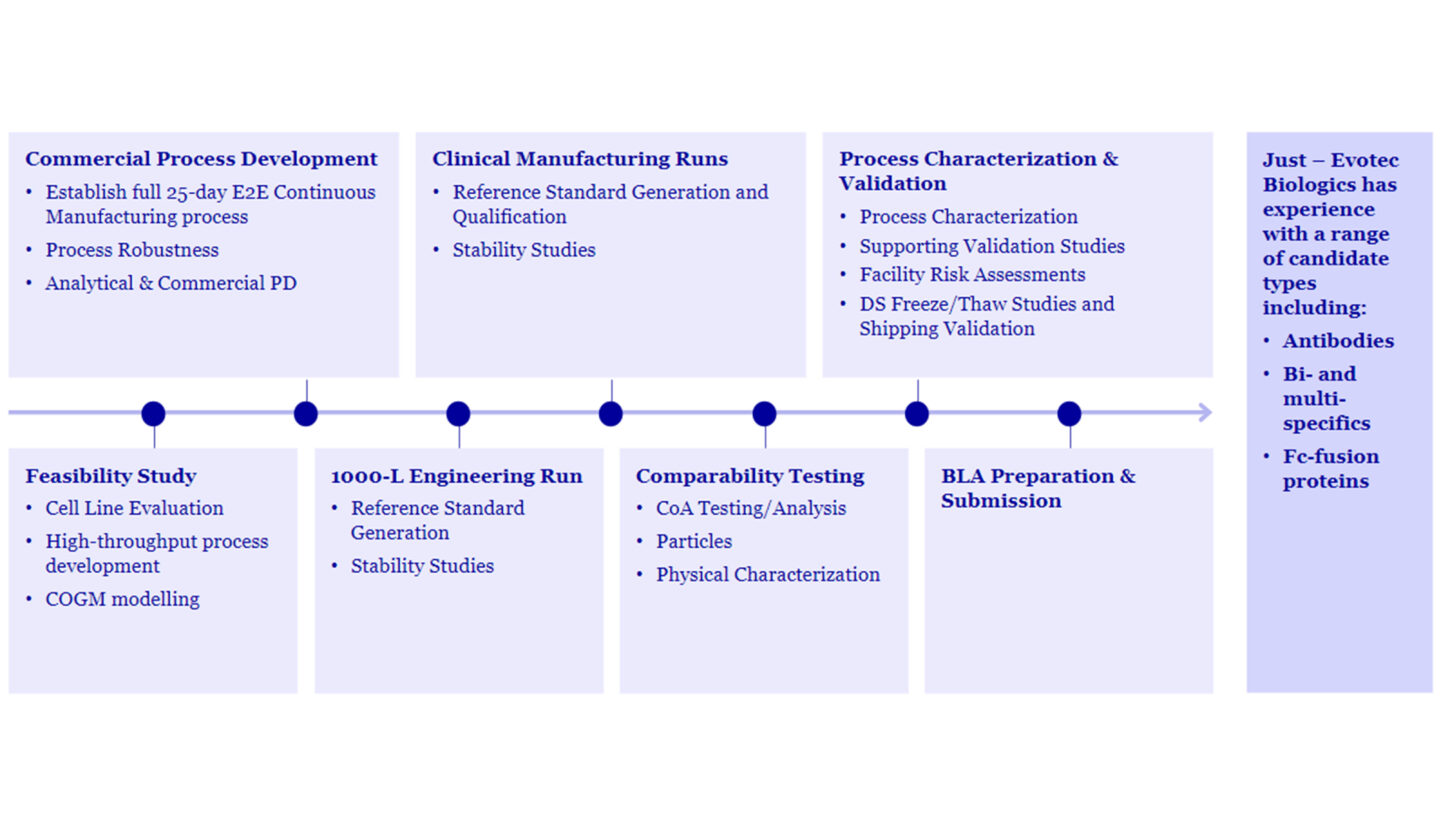
CPD at Just – Evotec Biologics fulfils three main functions:
- Establishing an End-to-End Continuous Manufacturing Process: Just – Evotec Biologics establishes a 25-day continuous manufacturing process for our partner’s molecule. This end-to-end approach ensures seamless production, from raw materials to drug substance with our automated manufacturing technology.
- Robustness Demonstration: The CPD team rigorously tests and validates the process with highly automated process development tools to ensure its robustness. This involves assessing factors like scalability, stability, and reproducibility. Robustness is critical for consistent product quality and reliable commercial supply.
- Commercial-Ready Analytical Strategy: Developing a robust analytical strategy is essential for commercial success. It involves methods for assessing product quality, purity, and safety. Just – Evotec Biologics uses analytical techniques that meet regulatory requirements and support commercial-scale production.
Late Phase Clinical Development & Regulatory Submissions
Just - Evotec Biologics provides partners with a clear pathway from Commercial Process Development through to BLA Submission to the FDA via the following steps:
- Scale-up and Engineering Runs
- Clinical Manufacturing
- Comparability Testing
- Process Characterization and Validation
- Biologics License Application Preparation and Submission
