Preclinical species hepatic Oatp transporter inhibition is in our portfolio of in vitro drug transporter services. Cyprotex delivers consistent, high quality data in line with our published transporter strategy review, for either your early stage screening projects or your later stage regulatory studies.
Introduction
Identifying potential inhibitors of preclinical species hepatic uptake transporters in vitro:
- The SLC (solute carrier) super family of transporter proteins transport a wide range of different solutes across biological membranes using diverse energy coupling mechanisms1.
- One of the most important human SLC transporters expressed in human liver is OATP1B1 which is responsible for the hepatic uptake and rate-determining elimination of a wide range of endogenous compounds and drugs that are substrates2.
- Species differences in drug transporters with regard to their tissue distribution, expression levels and substrate specificity can be problematic for preclinical cross-species extrapolation of drug disposition (clearance) and DDI potential to human.
- The use of in vitro cell test systems that each overexpress the major hepatic Oatp transporter of preclinical species (Oatp1b2, Oatp1b4 or Oatp1b1 for rat, dog or Cynomolgus monkey, respectively) may be useful towards understanding whether a molecule is an inhibitor of a hepatic active uptake transporter in those species. Whilst not usually performed for a compound on the back of IC50 data indicating a positive interaction potential for inhibition of human OATP1B1, were a sponsor to consider conducting an in vivo interaction study in a preclinical species (e.g. Cynomolgus monkey)3 then it would be beneficial to understand the in vitro inhibitory potential of the molecule against that preclinical species Oatp in order to put it into context with exposure and aid interpretation of the data.
- Cyprotex’s preclinical species hepatic Oatp transporter inhibition assay determines if your compound is an inhibitor of key preclinical species transporters.
Protocol
Preclinical Species Hepatic Oatp Transporter Inhibition Assay Protocol
Data
Data from Cyprotex's Preclinical Species Hepatic Oatp Transporter Inhibition Assay
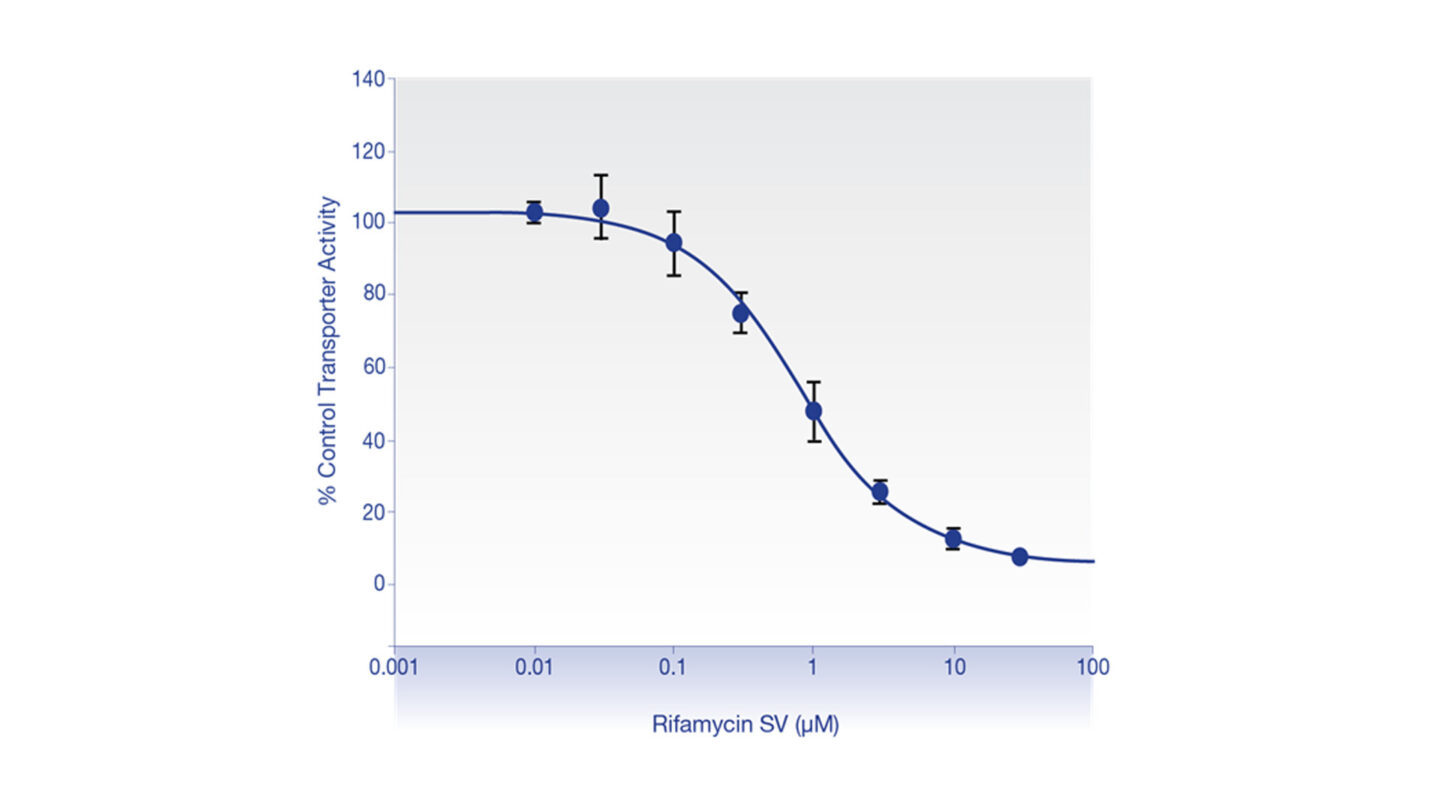
Figure 1
Mean rat Oatp1b2-mediated estradiol 17ß-D-glucuronide (1 μM) transport in the presence of a range of concentrations of rifamycin SV expressed as a percentage of vehicle control.
The results represent the mean ± standard deviation of 3-9 replicate wells (triplicate wells per incubation condition performed on three separate occasions).
References
1) Schlessinger A et al., (2013) Molecular modeling and ligand docking for solute carrier (SLC) transporters. Curr Top Med Chem 13(7): 843-856
2) Shitara Y et al., (2013) Clinical significance of organic anion transporting polypeptides (OATPs) in drug disposition: their roles in hepatic clearance and intestinal absorption. Biopharm Drug Dispos 34: 45-78
3) Ufuk A et al., (2018) In Vitro-In Vivo Extrapolation of OATP1B-Mediated Drug-Drug Interactions in Cynomolgus Monkey. J Pharmacol Exp Ther 365(3): 688-699
4) Chu X et al., (2013) Species differences in drug transporters and implications for translating preclinical findings to humans. Expert Opin Drug Metab Toxicol 9(3): 237-252
Downloads
- Cyprotex ADME Guide 5th Edition >
- Cyprotex DDI Regulatory Guide 3rd Edition >
- Cyprotex Transporter Strategy Guide >
- Cyprotex Preclinical Species Hepatic Oatp Inhibition Assay Factsheet >